The Amphibian Chytrid Fungus and Chytridiomycosis
What is a “chytrid”? What is Bd and Bsal?
The pandemic
Do all amphibians infected with Bd die?
How does the disease progress?
What can be done to help?
What can you do to help?
What are amphibian chytrid fungi?
Can populations with chytridiomycosis recover?
Where are Bd and Bsal found?
Where did they come from?
How does Bd spread?
What are the signs of chytridiomycosis?
How is chytridiomycosis diagnosed?
Can chytridiomycosis be treated?
What can you do to help?
Is Bd the greatest threat to amphibians?
Further reading
Literature cited
What is a “chytrid”? What is Bd and Bsal? Amphibian chytridiomycosis is an infectious fungal disease that can be fatal to amphibians. As the disease spread globally beginning in the 1970s, many populations declined greatly and species became extinct. This pandemic served as a first precedent for the threat of infectious diseases directly on biodiversity.
Chytridiomycosis is a skin disease in amphibians caused by either of two species of amphibian chytrid fungus. They are called Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans. Not surprisingly, everyone including the experts refer to them by their nicknames “Bd” and “Bsal.”
Both species of the amphibian chytrid fungus are native to southern Asia, but have been unintentionally introduced at various points around the world as part of global trade in amphibians as food, pets, or research animals. When these chytrid fungi enter ecosystems where they did not occur naturally, then sometimes epidemics of chytridiomycosis occur
Some epidemics of the disease, particularly in the 1980s through mid-2000s, have caused serious declines in native amphibian populations that, in some cases, have led to extinction of entire species. The worst epidemics, caused by Bd, were in Australia, Mexico and Central America, the Andes mountain chain of South America, and in the western US. Bsal currently is only known to affecting salamanders in western Europe, so efforts are being made to make sure it does not spread from there to other areas and species.
The global declines and extinctions of amphibians were first noticed, and misunderstood at the time, in the 1970s and 1980s. Bd was not even discovered until 1999 and it was not until the early 2000s that conservationists really had a grasp of the magnitude of the problem. This was the first time in the history of biodiversity conservation that experts realized that a disease can be a primary threat to biodiversity that can lead directly to extinction. As the world faces additional epidemics of infectious diseases, for example White-nose fungus in bats, we are realizing how much we learned from the amphibian crisis and hopefully applying our learned lessons to reduce the threats to these other groups.
Do all amphibians infected with Bd die? Not all amphibian species that are infected with Bd become sick or die. These species like the American bullfrog and the African clawed frog are said to be “resistant” to chytridiomycosis. Resistant species are a major concern because they are carriers of Bd (like a “Typhoid Mary”) that can move the fungus to new locations and expose new populations of amphibians that are “susceptible” or more likely to become sick with lethal chytridiomycosis. The reason why some amphibian species are resistant to chytridiomycosis is an area of very active scientific research. If we can understand why some species are resistant, it might be possible to develop methods to control chytridiomycosis in amphibian populations that experience devastating population declines. Some of the mechanisms that could explain species resistance to chytridiomycosis are:
- The presence on the skin of specific types of symbiotic bacteria that discourage the growth of Bd (Harris et al., 2009 a and b). Amphibians or amphibian populations that normally have large numbers of these bacteria in the skin might be more resistant to developing chytridiomycosis.
- The production by the poison glands in amphibian skin of chemicals called “antimicrobial peptides” that discourage the growth of Bd. Specific types, combinations or amounts of antimicrobial peptides might help some species to be more resistant to chytridiomycosis.
- Some amphibian species or populations may have genetic resistance to the development of chytridiomycosis by mechanisms that are not yet understood.
Other scientists study why some populations of amphibians succumb to chytridiomycosis while other populations of the same species persist. In addition to things like the presence of symbiotic bacteria or differences in skin peptide composition, some potential explanations include:
- Environmental differences between populations such as temperature, humidity or water flow patterns. For instance, some of the most important amphibian population declines associated with chytridiomycosis have occurred at high elevation locations that have a cool temperature range (< 250C or 770F) that is most optimal for the growth of Bd.
- Differences in virulence between different types or “strains” of the Bd fungus. The term virulence refers to the ability of the fungus to cause disease in amphibians. A type of Bd that is “highly virulent” easily makes amphibians sick, but another type of Bd that has “low virulence” makes fewer animals sick or results in less severe disease.
There is not a single explanation for why an amphibian population succumbs or does not succumb to chytridiomycosis and in most cases multiple factors are probably at work to result in a particular outcome.
How does the disease progress? These chytrid fungi are microscopic and grow in the skin layers of an infected amphibian. The fungi release spores that swim through the water until they settle on the skin of a new host. Infected animals may show no symptoms, or they may become sick with symptoms such as unhealthy skin and sluggishness, or they may die. Deaths are caused by heart failure that results from inability of the diseased amphibian to regulate the water moving through its permeable skin. Various factors influence how severe will be the effects of these chytrid fungi on with an individual amphibian or a population/species. These factors include the involved, the local environmental conditions, the level of infection (how many spores have infected and individual, or how many infected individuals are in a given area), as well as the genetic strain of the chytrid involved.
What can be done to help? Sick amphibians in human care may be treated by a veterinarian, but researchers have found no way to treat entire populations or species in the wild. Researchers also have not found a way to stop the spread of the disease in the wild, once it becomes established. With these realities in mind, conservationists are focused on reducing and regulating the global trade in amphibians and promoting biosecurity policies. Certainly, there are other diseases and parasites of amphibians that should not be given the chance to establish themselves in new areas of the world, so these are ongoing efforts aimed at the general protection of wild amphibians.
What can you do to help? The best way for a citizen to combat amphibian diseases is to make sure never to move amphibians between places. This also means, never releasing any amphibian that has been in captivity into the wild. If you decide to raise tadpoles, or have a pet frog or salamander, please consider that eventually releasing them is not recommended. Conservation programs, including those at Zoo Atlanta, that raise endangered species of amphibians for re-introduction to the wild do so with very high levels of biosecurity measures in place, to ensure that the released animals are free from infection.
What are amphibian chytrid fungi? Amphibian chytridiomycosis is a skin disease caused by microscopic fungal pathogens Batrachochytrium dendrobatidis or B. salamandrivorans, commonly known as “amphibian chytrid fungus” or by their nicknames “Bd” or “Bsal.” While there are over 1,000 species of chytrid fungi known, most live freely in the environment as decomposers of plant material. These two species, however, are unusual in that they are sometimes lethal parasites of amphibians.
The fungi disperse via zoospores that swim freely through water. The zoospores embed in the skin of amphibians and develop into a reproductive form called zoosporangium that digest proteins, especially keratin, in the skin and produce more zoospores that are released into the environment. The infected amphibian may tolerate the parasite and be essentially uninfected, or it may become diseased—a condition known as amphibian chytridiomycosis. The severity of the disease ranges from minimal to lethal and is dependent on many factors in the local environment, such as temperature, genetic strain of fungus involved, the number of chytrid organisms in the individual, or life-stage of the amphibian, or the identity of the species of the amphibian host.
Diseased animals show symptoms such as excessive shedding of skin, lethargy, and imbalance. The destruction of the skin can produce fatal imbalance of water and electrolytes in the body and lead to death from heart failure.
Chytrid zoospores may be transferred by amphibian-to-amphibian contact, but the usual mode of infection is from the free-swimming zoospores in the water or even wet surfaces such as rocks or soil. Once released into the water, the zoospores may simply re-infect the same individual or disperse to infect a new individual. Amphibian species may respond differently to chytrid infections. Some species, such as the American bullfrog (Lithobates catesbeianus) appear to be fully tolerant of even massive levels of infection, showing no signs of disease. Other species, such as the harlequin toads (genus Atelopus) of Central and South America are highly intolerant and usually die soon after infection. Larval animals, such as frog tadpoles, can become infected but they very rarely die from that infection before reaching metamorphosis. Thus, disease is more significant in adult-form individuals.
Most parasites may infect only a few species of host organisms, or a group of closely related species. Bd, however, is unusual in that it appears to be able to infect any of the more than 8000 known species of amphibian—even if the severity of the infection may vary widely among those species. This means that any individual amphibian can become infected and, regardless of the level of disease it may show, can be a carrier of the pathogen and spreader of disease.
In terms of conservation concerns, Bd has had its most devastating effects on frogs. Much less is known about Bsal, owing to its relatively recent discovery, but research is ongoing. Initial studies indicate that it can infect a variety of amphibian hosts, but its catastrophic epidemics seem to primarily affect salamanders.
The effects of epidemics of chytridiomycosis on populations and communities are quite complex. As in the case of an individual amphibian, the severity of local epidemics depends on many factors, including the local environment, the species involved, or the genetic strain of chytrid involved. Some populations or amphibian communities may tolerate chytrids and entirely avoid a disease epidemic, while others suffer severe epidemics that can lead to disappearance of entire populations.
Amphibian chytrid fungi are unusual among parasitic diseases in that they can cause extinction, sometimes rapidly, of entire species. A conservative estimate published in 2019 suggested that at least 90 species of amphibians have gone extinct in the last few decades and about 500 species are suffering catastrophic declines.
Can amphibian populations with chytridiomycosis recover? Some amphibian populations experience devastating mass mortality events due to chytridiomycosis where most of the population succumbs to the disease, but a small number of animals remain or “persist” in the population. At this time it is unknown if these “persistent populations” might eventually recover and regain the numbers of animals they had prior to chytridiomycosis or if these populations will remain small or even eventually disappear. Recent research has shown that a critical factor in determining if chytridiomycosis will cause extinction of an amphibian population is if the level of intensity of the infection with Bd crosses a certain threshold (Briggs et al., 2010; Vredenberg et al., 2010). What is very interesting about the “persistent” populations is that the remaining animals are still infected with Bd, but at a lower or less lethal intensity. Like the individual amphibian species that are resistant to chytridiomycosis (see above), understanding why persistent populations maintain low intensity infections with Bd is very important and could lead to methods to control the disease in wild populations.
Where are Bd and Bsal found? Where did they come from? Amphibian biologists in the late 1970s and through the 1980s watched many populations of frogs, and a few salamanders, decline drastically, or disappear completely; a few species evidently became extinct. The best-documented declines occurred in the western United State, eastern Australia, lower Central America, and in the northern Andes of South America. Although the devastating effects were obvious, the pathogen was unknown until Bd was finally discovered and described in 1999.
Ecologists working in Costa Rica and Panama, in the mid 2000’s demonstrated how local epidemics of Bd lead to population declines and extinctions and how they spread across landscapes. Around the same time, an effective test was developed and global patterns began to emerge. Bsal, however, was not discovered until 2013 when biologists realized that the decline of a salamander population in The Netherlands did not match the profile of a typical Bd epidemic.
Testing from field sites around the world, and from historical museum collections revealed that Bd was virtually everywhere, with the notable exception of Papua New Guinea, and very few records from Madagascar. We now know that Bd is native to southern Asia and was unwittingly spread globally by humans as trade in amphibians became more prevalent though the 20th Century. Amphibians began to be shipped by the millions for food, pets, and as research animals and they silently carried their fungal pathogens with them. Besides allowing non-native pathogens to entire naïve ecosystems, these patterns of trade set up a complex scenario in which different genetic strains of Bd came into contact and hybridized, creative formidably dangerous super-pathogens. Bsal also appears to be native in southern Asia and was introduced to western Europe, where it currently is devastating some salamander populations.
Bd has now been heavily studied in the lab and in the wild, while Bsal remains much less known. The worst aspects of the initial global pandemic of amphibian chytridiomycosis may have passed, and amphibian ecologists and conservationists are concentrating their efforts on populations that are struggling to recover. Thanks to lessons-learned from the original Bd epidemics, efforts to keep Bsal from spreading beyond western Europe have so far been effective. Nevertheless, future flare-ups of Bd and eventual spread of Bsal are very real possibilities and require constant vigilance.
How does Bd spread? Infection with Bd is transmitted by a form of the fungus called a “zoospore”. The zoospore has a very distinctive appearance with a single flagellum that helps the spore swim through water or moist environments. Zoospores require moisture and cool temperatures and can persist in moist environments for several months (Johnson and Speare, 2003), but do not tolerate conditions that are warm or dry for more than a few hours (Johnson and Speare, 2005). Therefore, the most common and successful ways that Bd zoospores spread from place to place are in water, moist or wet materials (including soil or equipment) or on the skin of infected amphibians. In fact, the most common way that Bd infection spreads between amphibians is from direct contact of an infected animal with an uninfected animal (e.g. during territorial or breeding encounters). In captivity, it is possible to house amphibians infected with Bd in enclosures next to enclosures with amphibians that are not infected with Bd and not transmit the infection as long as animals, water and wet materials and tools are not shared between the enclosures. Guidelines to reduce the transmission of Bd in captive environments are available (Pessier and Mendelson, 2010). In the natural environment, it has been hypothesized that Bd can move on people’s boots or equipment or on birds and invertebrates that fly between watersheds (Johnson and Speare, 2005). Therefore, it is important that biologists and others take precautions to clean and disinfect their boots and equipment before moving from one location that has amphibians to another location in order to minimize the risk of spreading Bd (Phillot et al., 2010). Because many amphibians that are infected with Bd are resistant to the disease chytridiomycosis (see above), they can appear to be outwardly healthy but are still capable of spreading Bd from one location to another. This is important because these animals may act as a reservoir for transmitting Bd infection to other amphibians as part of natural movements between different watersheds. Amphibians can also move Bd to new locations as the result of trade in amphibians (see above) or potentially by the release of captive amphibians to the wild (See Amphibians in Classrooms).
What are the signs of chytridiomycosis? An amphibian that is sick with chytridiomycosis can have a wide variety of symptoms or “clinical signs”. Some of the most common signs are reddened or otherwise discolored skin, excessive shedding of skin, abnormal postures such as a preference for keeping the skin of the belly away from the ground, unnatural behaviors such as a nocturnal species that suddenly becomes active during the day, or seizures. Many of these signs are said to be “non-specific” and many different amphibian diseases have signs that overlap with those of chytridiomycosis. In addition, other cases of chytridiomycosis will not show any of these signs and amphibians will simply be found dead. For these reasons it is not possible to diagnose chytridiomycosis with the naked eye and laboratory testing is required (see How is Chytridiomycosis Diagnosed? below).
How is chytridiomycosis diagnosed? 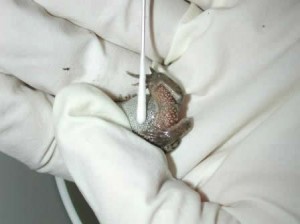 If animals are sick it is possible to diagnose chytridiomycosis by examining samples of the skin under a microscope and identifying the characteristic fungal organisms of Bd. These techniques require the assistance of an experienced biologist or veterinarian and are not good ways to detect amphibians that are carriers of Bd. Alternatively, non-invasive swabs of the skin can be obtained and analyzed by a technique called the polymerase chain reaction or “PCR” for short (Hyatt et al., 2007). PCR can detect very small amounts of Bd DNA in a sample and for this reason it is the test of choice for detecting animals that carry Bd infection and to survey wild and captive amphibian populations for the presence of Bd. Check out a video clip that demonstrates the collection of samples for Bd PCR. More information about sampling techniques (in English and in Spanish) can be found on the AmphibiaWeb site. A complete discussion of different diagnostic methods for Bd can be found in Pessier and Mendelson, 2010.
If animals are sick it is possible to diagnose chytridiomycosis by examining samples of the skin under a microscope and identifying the characteristic fungal organisms of Bd. These techniques require the assistance of an experienced biologist or veterinarian and are not good ways to detect amphibians that are carriers of Bd. Alternatively, non-invasive swabs of the skin can be obtained and analyzed by a technique called the polymerase chain reaction or “PCR” for short (Hyatt et al., 2007). PCR can detect very small amounts of Bd DNA in a sample and for this reason it is the test of choice for detecting animals that carry Bd infection and to survey wild and captive amphibian populations for the presence of Bd. Check out a video clip that demonstrates the collection of samples for Bd PCR. More information about sampling techniques (in English and in Spanish) can be found on the AmphibiaWeb site. A complete discussion of different diagnostic methods for Bd can be found in Pessier and Mendelson, 2010.
PCR requires a molecular biology laboratory that uses rigorous controls for positive and negative samples and that has carefully validated the PCR test. A disadvantage of PCR is that it is not able to distinguish between amphibians that are sick with chytridiomycosis and amphibians that are carriers of Bd, because both types of animals will test “positive” by PCR.
Can chytridiomycosis be treated? In captive amphibians, chytridiomycosis can be successfully treated with antifungal medications and by disinfection of contaminated enclosures (Pessier and Mendelson, 2010). A variety of different antifungal medications have been described for the treatment of chytridiomycosis, however, one of the most common methods was developed at the Smithsonian National Zoo and uses a series of baths in the drug itraconazole (Nichols and Lamirande, 2000). Itraconazole baths have been used successfully in rescue operations that capture wild amphibians from populations that are experiencing deaths to chytridiomycosis (Gagliardo et al., 2008). Other potential treatment methods include the use of elevated body temperature and paradoxically, the antibiotic chloramphenicol. Treatment is not always 100% successful and not all amphibians tolerate treatment very well, therefore chytridiomycosis should always be treated with the advice of a veterinarian. Unfortunately, there are no good methods for the treatment of wild animals in the natural environment. It is very difficult or impossible to get enough of the antifungal medications into the environment to be able to successfully rid infected frogs of Bd. In the future it may be possible to treat some amphibians in the wild in order to reduce the intensity of infection to a less lethal level with the hope that animals could survive with a mild Bd infection (Briggs et al., 2010; Vrendenberg et al., 2010). Another promising area of research is looking at the possibility of introducing symbiotic bacteria that inhibit the growth of Bd into wild amphibian populations (Harris et al., 2009). So far, there is no evidence that a vaccine for chytridiomycosis could be effective for controlling the disease in wild populations (Stice and Briggs, 2010).
What can you do to help? Emerging infectious diseases of wildlife are perhaps the most difficult of conservation challenges to address. The most important thing to realize is how this situation was created—by the legal and illegal trade in animals, with no regards to biosecurity precautions. Please support efforts to control and regulate all forms of trade in wildlife and, most importantly, never release into the wild any animal that has been in commercial trade. The best pro-active means we have of minimizing threats of chytrid fungi, and other pathogens, in the wild is to minimize trade in wild animals.
If you work with amphibians in any context or have pet amphibians, follow basic biosecurity and sanitation protocols to avoid moving chytrids between populations. Finally, realize that chytridiomycosis is certainly not the only threat that amphibians face, so all efforts to minimize negative effects of human activities on the environment will certainly help amphibians recover from past epidemics and hopefully avoid subsequent events.
Is Bd the greatest threat to amphibians? No. Habitat loss affects more amphibian species than any other threat by nearly a factor of 4. However, while habitat loss proceeds at a steady pace, Bd can often work quickly. The IUCN has called amphibian chytridiomycosis “the worst infectious disease ever recorded among vertebrates in terms of the number of species impacted, and its propensity to drive them to extinction.” And because the Amphibian Ark focuses on species facing threats that cannot currently be mitigated in the wild, such as Bd, we necessarily focus largely on this disease and leave the mitigable threats, such as habitat loss, to our ASA partners specializing in those areas.
Collins, J. P., and M. L. Crump. 2009. Extinction in our Times: Global Amphibian Decline. Oxford University Press, USA. 304pp.
Developing a safe antifungal treatment protocol to eliminate Batrachochytrium dendrobatidis from amphibians – A. MARTEL, P. VAN ROOIJ, G. VERCAUTEREN, K. BAERT, L. VAN WAEYENBERGHE, P. DEBACKER, T. W. J. GARNER, T. WOELTJES, R. DUCATELLE, F. HAESEBROUCK & F. PASMANS Murray, K., Skerratt, L., Marantelli, G., Berger, L., Hunter, D., Mahony, M. and Hines, H. 2011.
Guidelines for minimising disease risks associated with captive breeding, raising and restocking programs for Australian frogs. A report for the Australian Government Department of Sustainability, Environment, Water, Population and Communities.
Field-Sampling Protocol for Batrachochytrium dendrobatids From Living Amphibians, using Alcohol Preserved Swabs – Brem, Mendelson and Lips
A guide to husbandry and biosecurity standards required for the safe and responsible management of ex situ populations of amphibians These standards are based upon those reported in the proceedings of the CBSG/WAZA Amphibian Ex situ Conservation Planning Workshop, El Valle, Panama, 12-15th February 2006.
Andreone, F., A. I. Carpenter, N. Cox, L. du Preez, S. Furrer, G. Garcia, F. Glaw, J.Glos, D. Knox, J. Köhler, J. R. Mendelson III, V. Mercurio, R. A. Mittermeier, R. D. Moore, N. H. C. Rabibisoa, H. Randriamahazo, H. Randrianasolo, N. R.Raminosoa, O. R. Ramilijaona, C. J. Raxworthy, D. Vallan, M. Vences, D.R. Vieites, and C. Weldon. 2008. The challenge of conserving amphibian megadiversity in Madagascar. Public Library of Science Biology 6:1–4.
Berger, L., R. Speare, P Dazsak, D.E. Green, A.A. Cunningham, C.L. Goggin, R. Slocombe, M.A. Ragan, A.D. Hyatt, K.R. McDonald, H.B. Hines, K.R. Lips, G. Marantelli and H. Parkes . 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Sciences of the United States of America 95: 9031-9036.
Berger, L, R. Speare, H. Hines, G. Marantelli, A. D. Hyatt, K. R. McDonald, L. F. Skerratt, V. Olsen, J. M. Clarke, G. Gillespie, M. Mahony, N. Sheppard, C. Williams, and M. Tyler. 2004. Effect of season and temperature on mortality in amphibians due to chytridiomycosis. 2004. Australian Veterinary Journal 82 434–439.
Bradford, D. F. 1991. Mass mortality and extinction in a high-elevation population of Rana muscosa. Journal of Herpetology 25: 174–177.
Carey, C. 1993. Hypothesis concerning the causes of the disappearance of Boreal toads from the mountains of Colorado. Conservation Biology 7:355–362.
Carey, C., Heyer, W.R., J. Wilkinson, R. A. Alford, J. W. Arntzen, T. Halliday, L. Hungerford, K. R. Lips, E. M. Middleton, S. A. Orchard, and S. Rand, S. 2001 Amphibian declines and environmental change: use of remote sensing techniques to identify environmental correlates. Conservation Biology 15:909–913.
Collins, J. P., and A. Storfer. 2003. Global amphibian declines: sorting the hypotheses. Diversity and Distribution 9: 89–98.
Collins, J. P., and M. L. Crump. 2009. Extinction in our Times: Global Amphibian Decline. Oxford University Press, USA. 304pp.
Connelly, S., R. J. Bixby. C. M Pringle, R. Brenes, M. R. Whiles, K. R. Lips, S. Kilham, and A. D. Huryn. 2008. Changes in stream primary producer communities resulting from loss of tadpoles: can small-scale experiments predict effects of large-scale catastrophic amphibian declines? Ecosystems DOI: 10.1007/s10021-008-9191-7
Crump, M. L., F. R. Hensley, and K. L. Clark. 1992. Apparent decline of the golden toad: underground or extinct? Copeia 1992: 413–420.
Crump, M. L. 2000. In Search of the Golden Frog. University of Chicago Press, 320p.
Daszak, P., L. Berger, A. A. Cunningham, A. D. Hyatt, D. E. Green, and R. Speare. 1999. Emerging infectious diseases and amphibian population declines. Emerging Infectious Diseases 5: 735–748.
Fisher, M.C., T.W.J. Garner, and S.F. Walker. 2009. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annual Review of Microbiology 63:291–310.
Gagliardo, R., P.Crump , E. Griffith,et al. 2008. The principles of rapid response for amphibian conservation using the programmes in Panama as an example, International Zoo Yearbook 42: 125-135.
Garner T.W.J., M. Perkins, P. Govindarajulu, D. Seglie, S.J. Walker, A.A. Cunningham, and M.C. Fisher. 2006. The emerging amphibian pathogen Batrachochytrium dendrobatidis globally infects introduced populations of the North American bullfrog, Rana catesbeiana. Biol. Letters 2:455-459.
Gascon C., J.P. Collins, R.D. Moore et al., editors: Amphibian Conservation Action Plan. IUCN/SSC Amphibian Specialist Group. Gland, Switzerland and Cambridge UK, 2007.
Grant, E. H. C., et al. (28 co-authors). 2015. Salamander chytrid fungus (Batrachochytrium salamandrivorans) in the United States—Developing research, monitoring, and management strategies. U.S. Geological Survey Open-File Report 2015–1233, 16 p., http://dx.doi.org/10.3133/ofr20151233.
Heyer, W. R., A. S. Rand, C. A. G. da Cruz, and O. L. Peixoto. 1988. Decimations, extinctions, and colonizations of frog populations in southeast Brazil and their evolutionary implications. Biotropica 20: 230–235.
Kilpatrick, A. M., C. J. Briggs, and P. Daszak. 2009. The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends in Ecology and Evolution 1170: 1–11.
La Marca, E., K. R. Lips, S. Lötters, R. Puschendorf, R. Ibáñez, S. Ron, J. V. Rueda-Almonacid, R. Schulte, C. Marty, F. Castro, J. Manzanilla-Puppo, J. E. García-Pérez, M. R. Bustamante, L. A. Coloma, A. Merino-Viteri, E. Toral, F. Bolaños, G. Chaves, A. Pounds, B. Young. 2005. Catastrophic population declines and extinctions in Neotropical Harlequin frogs (Bufonidae: Atelopus). Biotropica 37:190–201.
Lannoo, M. (Editor). 2005. Amphibian Declines: The Conservation Status of United States Species. University of California Press, 1115p.
Laurance, W. F., K. R. McDonald, R. Speare. 1996. Epidemic disease and the catastrophic decline of Australian rain forest frogs. Conservation Biology 10: 406–413.
Lips, K. R. 1998. Decline of a Tropical Amphibian Fauna. Conservation Biology 12:106–117.
Lips, K. R. 1999. Mass mortality of the anuran fauna at an upland site in Panama. Conservation Biology 13: 117–125.
Lips, K. R., J. Reeve, and L. R. Witters. 2003. Ecological factors predicting amphibian population declines in Central America. Conservation Biology 17: 1078–1088.
Lips, K. R., J. R. Mendelson III, A. Muñoz-Alonso, L. Canseco-Márquez, and D. G. Mulcahy. 2004. Amphibian population declines in montane southern Mexico: resurveys of historical localities. Biological Conservation 119:555–564.
Lips, K. R., P. A. Burrowes, J. R. Mendelson III, and G. Parra-Olea. 2005. Amphibian declines in Latin America: widespread population declines, extinctions and impacts. Biotropica 37:163–165.
Lips, K. R., P. A. Burrowes, J. R. Mendelson III, and G. Parra-Olea. 2005. Amphibian declines in Latin America: a synthesis. Biotropica 37: 222–226.
Lips, K. R., and M. A. Donnelly. 2005. What the Topics can tell us about declining amphibian populations: current patterns and future prospects, pp. 388–406 In M. J. Lannoo (Editor). Amphibian Declines: The Conservation Status of United States Species. University of California Press, 1115p.
Lips, K. R., F. Brem, R. Brenes, J. D. Reeve, R. A. Alford, J. Voyles, C. Carey, A. Pessier, L. Livo, J. P. Collins. 2006. Infectious disease and global biodiversity loss: pathogens and enigmatic amphibian extinctions. Proceedings of the National Academy of Science (USA) 103: 3165–3170.
Lips, K. R., J. Diffendorfer, J. R. Mendelson III, and M. W. Sears. 2008. Riding the wave: reconciling the roles of disease and climate change in amphibian declines. Public Library of Science Biology 6: 1–15.
Lips, K. R., and J. R. Mendelson III. 2014. Stopping the next amphibian apocalypse. New York Times (invited Op-Ed). 14 November 2014.
Longcore, J.E., A.P. Pessier and D.K. Nichols. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91:219-227.
McMenamin, S. K., E. A. Hadly, and C. K. Wright. 2008. Climatic change and wetland dessication cause amphibian decline in Yellowstone National Park. Proceedings of the National Academy of Sciences (USA) 105: 16988–16993.
Mendelson, J. R., III et al. (plus 49 co-authors). 2006. Policy Forum: Confronting amphibian declines and extinctions. Science 313: 48.
Mendelson, J. R., III, J. B. Pramuk, R. Gagliardo, A. Pessier, B. B. Rothermel, K. C. Zippel, C. Bevier, M. Preest, and B. Crother. 2009. Considerations and recommendations for raising live amphibians in classrooms. Herpetological Review 40: 142–144.
Mendelson, J. R., III. 2011. Shifted baselines, forensic taxonomy, and Rabbs’ fringe-limbed treefrog: The changing role of biologists in an era of amphibian declines and extinctions. Herpetological Review 42: 21–25.
Mendelson, J. R., III, and R. Donnelly. 2011.The Crisis of Global Amphibian Declines: Causes, Consequences, and Solutions. Network for Conservation Educators and Practitioners, American Museum of Natural History. CD-ROM. System requirements: IBM PC or Mac compatible. Windows 98 or higher. Also available electronically at: (PowerPoint Teaching Tutorial, plus associated pedagogical materials) 97+ pp. http://research.amnh.org/biodiversity/ncep
Mendelson, J. R., III, and R. L. Hill. 2013. Amphibian skin swabbing procedure for Bd fungus. Online video tutorial: https://www.youtube.com/watch?v=a5CtPrGOK8c
Mendelson, J. R., III, M. E. B. Jones, A.P. Pessier, G. Toledo, E. H. Kabay, and J. A. Campbell. 2014. On the timing of an epidemic of amphibian chytridiomycosis in the highlands of Guatemala. South American Journal of Herpetology 9: 151–153.
Mendelson III, J.R., S. M. Whitfield, and M. J. Sredl. 2019. A recovery engine strategy for amphibian conservation in the context of disease. Biological Conservation 236:188–191
Mohneke, M., A. B. Onadeko, and M. O. Rödel. 2009. Exploitation of frogs—a review with a focus on West Africa. Salamandra 45: 193–202.
Nichols, D. K. 2003. Tracking down the killer chytrid of amphibians. Herpetological Review 34: 101–104.
Pechmann, J. H. K., D. E. Scott, R. D. Semlitsch, J. P. Caldwell, L. J. Vitt and J. W. Gibbons. 1991. Declining amphibian populations: the problem of separating human impacts from natural fluctuations. Science 253: 892–895.
Pessier, A. P. 2008. Management of disease as a threat to amphibian conservation. International Zoo Yearbook 42: 30–39.
Pessier, A.P. and J.R. Mendelson (eds.). 2010. A Manual for Control of Infectious Diseases in Amphibian Survival Assurance Colonies and Reintroduction Programs. IUCN/SSC Conservation Breeding Specialist Group: Apple Valley, MN.
Pilliod, D.S., E. Muths, R.D. Scherer, P.E. Bartelt, P.S. Corn, B.R. Hossack, B.A. Lambert, R.McCaffery, and C.G. Gaughan. 2010. Effects of amphibian chytrid fungus on individual survival probability in wild boreal toads. Conservation Biology 24: 1259–1267.
Rabb, G. B. 1990. Declining amphibian populations. Species, 13–14:33–34.
Rachowicz L. J., R. A. Knapp, J. A. T. Morgan, M. J. Stice, V. T. Vredenburg, J. M. Parker, and C. J. Briggs. 2006. Infectious disease as a proximate cause of amphibian mass mortality. Ecology 87: 1671–1683.
Ranvestel, A. W., K. R. Lips, C. M. Pringle, M. R. Whiles, and R. J. Bixby. 2004. Neotropical tadpoles influence stream benthos: evidence for the ecological consequences of decline in amphibian populations. Freshwater Biology 49: 274–285.
Rohr, J. R., T. R. Raffel, J. Romansic, H. McCallum, and P. J. Hudson, P.J. 2008. Evaluating the links between climate, disease spread, and amphibian declines. Proceedings of the National Academy of Science (USA) 45: 17436–17441
Rosenblum, E. B., J. Voyles, T. J. Poorten, and J. E. Stajich. 2009. The deadly chytrid fungus: a story of an emerging pathogen. Public Library of Science Pathogens 6: e1000550.
Ryan, M. J., K. R. Lips, M. W. Eichholz. 2008. Decline and extirpation of an endangered Panamanian stream frog population (Craugastor punctariolus) due to an outbreak of chytridiomycosis. Biological Conservation 141: 1636–1647.
Scheele, B. C., et al. (39 co-authors). 2019. The aftermath of amphibian fungal panzootic reveals unprecedented loss of biodiversity. Science 363:1459–1463
Schloegel, L. M., J. M. Hero, L. Berger, R. Speare, K. McDonald, P. Daszak. 2006. The decline of the Sharp-snouted day frog (Taudactylus acutirostris): the first documented case of extinction by infection in a free-ranging wildlife species? Ecohealth 3:35–40.
Schloegel, L.M., A.M. Picco, A.M. Kilpatrick, A.J. Davies, A.D. Hyatt, and P. Daszak. 2009. Magnitude of the US trade in amphibians and the presence of Batrachochytrium dendrobatidis and Ranavirus infection in imported North American bullfrogs (Rana catesbeiana). Biological Conservation 142:1420-1426.
Seimon, T. A, A. Seimon, P. Daszak, S. R. P. Halloy, L. M. Schloegel, C. A. Aguilar, P. Sowell, A. D. Hyatt, B. Konecky, and J. E. Simmons. 2007. Upward range extension of Andean anurans and chytridiomycosis to extreme elevations in response to tropical deglaciation. Global Change Biology 13: 288–299.
Semlitsch, R. D. (Editor). 2003. Amphibian Conservation. Smithsonian Institution Press, 336p.
Stuart, S., M. Hoffman, J. Chanson, N. Cox, R. Berridge, P. Ramani, and B. Young (Editors) 2008. Threatened Amphibians of the World, Lynx Ediciones, Barcelona, Spain, 758pp.
Vial, J.L. 1991. Declining Amphibian Populations Task Force. Species 16:47–48.
Voyles, J., L. Berger, S. Young, R. Speare, R. Webb, J. Warner, D. Rudd, R. Campbell, and L. F. Skerratt. 2007. Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis. Diseases of Aquatic Organisms 77: 113–118.
Voyles, J., S. Young, L. Berger, C. Campbell, W.F. Voyles, A. Dinudom, D. Cook, R. Webb, R.A. Alford, L.F. Skerratt, and R. Speare. 2009. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326:582-585.
Vredenberg, V. T., R. A. Knapp, T. S. Tunstall, and C. J. Briggs. 2010. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proceedings of the National Academy of Sciences (USA): doi/10.1037/pnas.0914111107
Wake, D. B. 1991. Declining amphibian populations. Science 253: 860.
Whiles, M. R., K. R. Lips, C. Pringle, S. S. Kilham, R. Brenes, S. Connelly, J. C. Colon Guad, M. Hunte-Brown, A. D. Huryn, C. Montgomery, and S. Peterson. 2006. The consequences of amphibian population declines to the structure and function of neotropical stream ecosystems. Frontiers in Ecology and the Environment 4: 27–34.
Whitfield, S. M., K. E. Bell, T. Philippi, M. Sasa, F. Bolaños, G. Chaves, J. M. Savage, and M. A. Donnelly. 2007. Amphibian and reptile declines over 35 years at La Selva, Costa Rica. Proceedings of the National Academy of Sciences (USA) 104: 8352–8356.
Wells, K. D. 2007. The Ecology and Behavior of Amphibians. University of Chicago Press, 1148p.
Woodhams, D. C., R. A. Alford, and G. Marantelli. 2003. Emerging disease of amphibians cured by elevated body temperature. Diseases of Aquatic Organisms 55:65–67.








